- TAVR Transcatheter Aortic Valve Replacement
- TAVr Transcatheter Aortic Valve repair
- TAVR Transcatheter Aortic Valve Replacement
- TAVr Transcatheter Aortic Valve repair
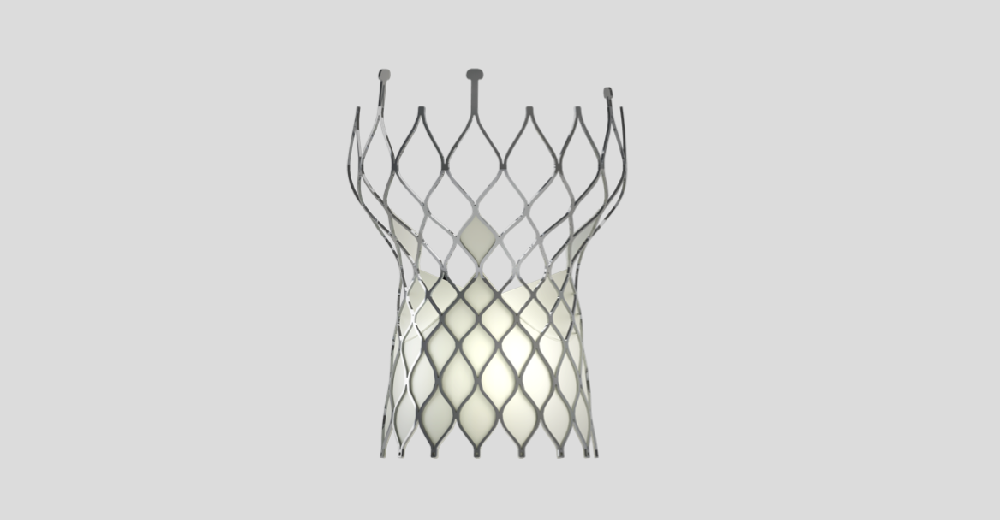
VenusA-Valve
Transcatheter Aortic Valve Replacement System
VenusA-Valve
VenusA-Valve is the first transcatheter aortic valve replacement system approved in China. Its unique stent design enables it to work without balloon dilation and segmental release ensures a simple and stable operation process. In particular, radial force is enhanced for the higher frequency of bicuspid aortic valve and heavy calcification in Chinese patients.
VenusA-Valve was approved by China National Medical Products Administration (NMPA) on April 25th, 2017. It is the first transcatheter aortic valve replacement system commercialized in China. VenusA-Valve has dominated the country’s TAVR segment since day one with a market share consistently above 80%.
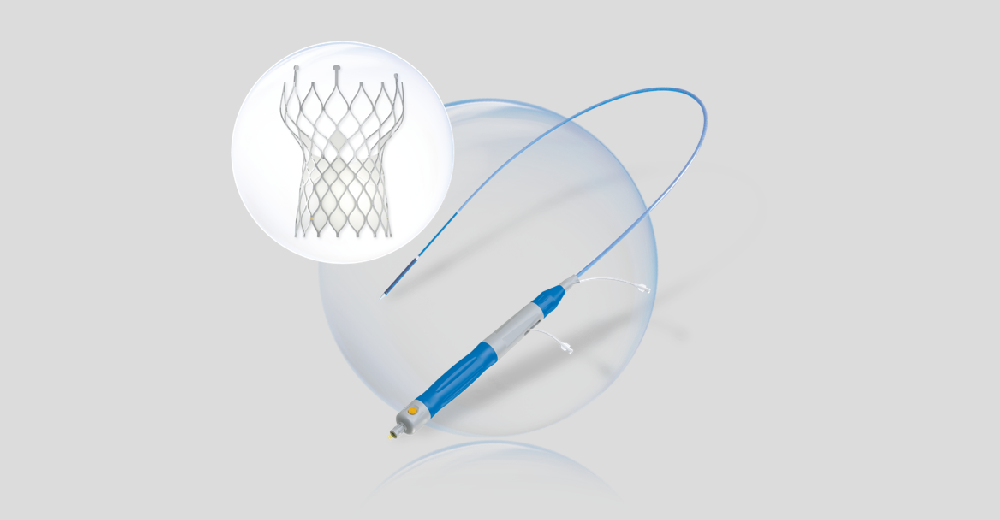
VenusA-Plus
Transcatheter Aortic Valve Replacement System – Retrievable Delivery System
VenusA-Plus
VenusA-Plus retains the advantage of strengthened radial force of VenusA-Valve, the first-generation valve replacement system of the Company, while thoroughly optimizing the delivery system and adding retrievable and repositionable features. This would effectively decrease the difficulty, promote successful rate, and improve postoperative benefits. VenusA-Plus will shorten the learning curve of doctors and help accelerate the development and popularization of TAVR in China.
VenusA-Plus was approved by NMPA on November 10th, 2020 and was commercially applied in the same year. On December 18th, 2020, VenusA-Plus was registered and approved by the Food and Drug Administration Committee of the Ministry of Public Health of Thailand.

VenusA-Plus

Venus-Vitae
Next-Generation Aortic Valve Replacement System– Balloon-Expandable
Venus-Vitae
Venus-Vitae is the next-generation balloon-expandable transcatheter heart valve system developed by Venus Medtech, with global patent protection. Venus-Vitae utilizes the innovative Venus-Endura technology, which integrates multiple anti-calcification techniques, immunogenicity removal technology and 3D force-controlled dehydration technology, providing exceptional durability, biocompatibility, and excellent anti-calcification performance. In addition, this design allows for room temperature storage of the valve in a dry-tissue condition.
Additionally, the product features a unique patented technology, Lockwire Technology, ensuring the stability and accuracy of the valve during delivery and deployment. The short stent frame of Venus-Vitae's valve along with its steerable NAVIMASTER delivery system, significantly optimizes the performance across the aortic arch and positioning within the native valve. Venus-Vitae also features a unique Coronary Alignment technology, addressing the fundamental challenge that current TAVR products have been unable to address—aiding in protection of the coronary arteries. By utilizing three gold markers at the base of the valve leaflets in conjunction with the delivery system's steering, telescoping, and balloon rotation capabilities, Venus-Vitae can effortlessly and precisely achieve coronary alignment during the deployment process. This aides in accomplishing coronary protection throughout the implantation procedure.
Furthermore, Venus-Vitae features a self-adaptive anti-PVL technology, utilizing an adaptive polymer skirt. The proprietary polymer skirt material possesses a high compression ratio, excellent resilience, self-expanding properties, and adaptive sealing. It demonstrates adaptive deformation during valve crimping, without enlarging the profile of the delivery system. Upon deployment, it actively fills the gaps around the valve perimeter, achieving remarkable anti-PVL performance.
Venus-Vitae was granted approval in Argentina and Chile in 2022 and 2023 in succession.
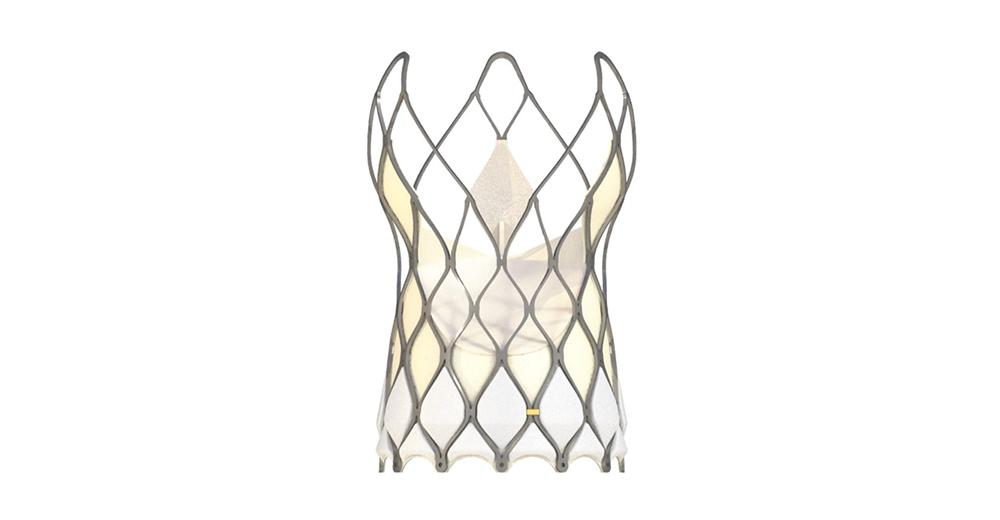
Venus-PowerX
Next-Generation Aortic Valve Replacement System– Self-Expanding
Venus-PowerX
Venus-PowerX is Venus Medtech’s next-generation self-expanding dry-tissue TAVR system for the treatment of patients with aortic valve stenosis. Venus-PowerX utilizes the innovative Venus-Endura technology, which integrates multiple anti-calcification techniques, immunogenicity removal technology and 3D force-controlled dehydration technology, providing exceptional durability, biocompatibility, and excellent anti-calcification performance. In addition, this design allows room temperature storage of the valve in a dry-tissue condition.
Venus-PowerX features a unique wire-controlled function that allows for full retrieval of the valve after 100% release which enhances the safety of implantation. In addition, the PowerX Delivery System adopts a new design that optimizes its flexibility to go through the aortic arch. The three V-shaped outflow portions of the valve are designed to preserve space for coronary access post implant, whilst also retaining excellent radial force.
Venus-PowerX's self-adaptive anti-PVL technology utilizes an adaptive polymer skirt. The proprietary polymer skirt material possesses a high compression ratio, excellent resilience, self-expanding properties with adaptive sealing. It demonstrates adaptive deformation during valve crimping, without enlarging the profile of the delivery system. Upon deployment, it actively fills the gaps around the valve perimeter, achieving remarkable anti-PVL performance.
Venus-PowerX was granted approval in Argentina and Chile in 2023 successively.

Venus-PowerX
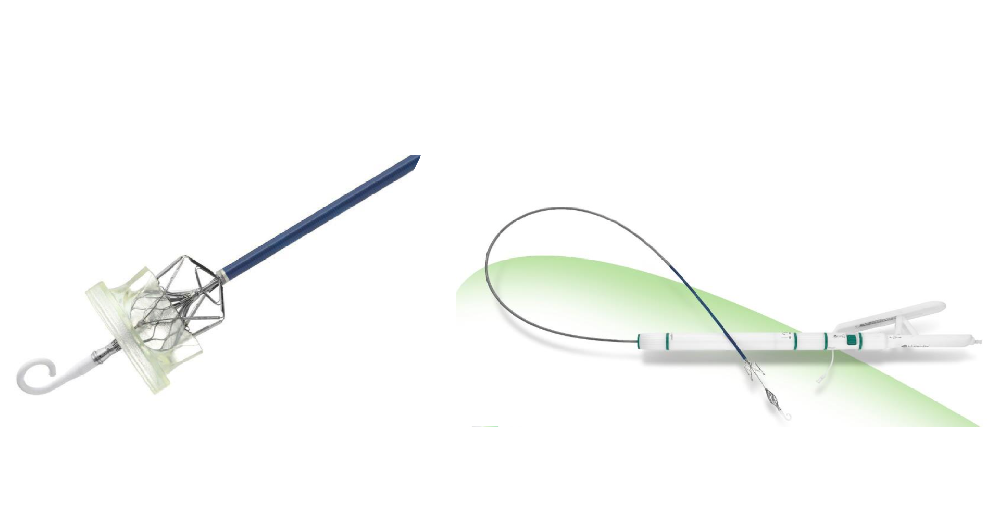
Leaflex™ Performer
Transcatheter Aortic Valve System
Leaflex™ Performer
Leaflex™ catheter performs mechanical scoring of valve calcification, restoring leaflets' mobility and improving valve hemodynamics.
The product was developed by Pi-Cardia Ltd. (Pi-Cardia), a global leader in the development of non-implant catheter-based solutions for treating heart valve calcification, and was introduced to China by Venus Medtech in September 2020. In October 2021, Leaflex™ completed its FIM (First-in-man) clinical trial in China.
The Leaflex™ catheter is designed to be a cost-effective, durable standalone treatment. It can be used for patients who are not planning to undergo transcatheter aortic valve replacement (TAVR) and it can be a means to defer TAVR in patients who may be too young for the procedure. It can also be a preparatory step for improving the outcome of TAVR in heavily-calcified and bicuspid aortic valves. Leaflex is also applicable for patients for those who’ve implanted a prosthetic heart valve for years. Affordable solution with short hospitalization is of great significance for patients suffering from aortic stenosis